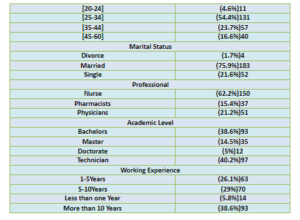
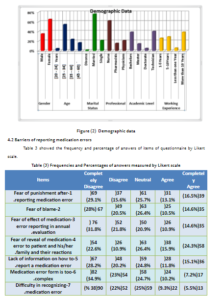
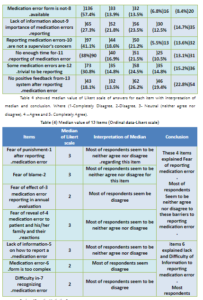
Barriers of Reporting Medication Errors at Arar Central Hospital, KSA
Abdulealah A. Alarfj
Riyadh Elm University || Saudi Arabia
معوقات الإبلاغ عن الأخطاء الدوائية في مستشفى عرعر المركزي
بالمملكة العربية السعودية
عبد الإله عايد العرفج
جامعة رياض العلم || المملكة العربية السعودية
-
Introduction:
Medication errors (MEs) are the most common types of medical errors harming about 1.5 million people, kill 107000 people (Aspden et al., 2006). An estimated number of 48000 – 99000 patients die from medical errors each year. About 7000 people annually are expected to die from medication error (Phillips et al., 1998). Dr. Makary and Daniel estimated that “medical errors are third cause of death in US” (Makary and Daniel, 2016). Medication errors also oblige significant costs between 6 billion US $ and 29 billion US $ per year (WHO, 2014) and cost around 3.5 billion US $ yearly (Aspden et al., 2006).
Adverse drug events (ADEs) and medication errors (MEs) are frequent in health care institutions and can happen at any step in the medication use process. Medication errors are most common than ADEs. Depending on the setting, about a third to half of ADEs are typically related to medication errors (Leape et al., 1993). Adverse drug events (ADEs) considered the most cause of harm to hospitalized patients (Samsiah et al., 2016). Medication errors and adverse drug events are a continual source of error in healthcare and associated with the increased illness of patients and cost (Roughead and Semple 2009). Although the rate of medication errors was widely different as a result of a difference in definition and detecting methods of medication errors, it has given alarming of medication errors problem.
Medication errors are drawing attention of the organizations, agencies, quality institutions worldwide as one of the most critical challenges in front of patient safety. World Health Organization announced that “Medication without harm” is WHO’s Third Global Patient Safety Challenge, Medication without Harm aims to reduce severe avoidable medication-related harm by 50%, globally in the next five years (Donaldson et al. 2017). An error will occur, and Human error is unavoidable. Nothing to be done to eliminate human errors. To effectively avoid the mistake that can cause patient harm, improvement system problems is required. System problem can be visible through reports all errors harming patients, errors that reached patient but do not result in patient harm, and errors that were prevent before reached patient (Wolf and Hughes, 2008).
Reporting errors is the most common method of learning and preventing future errors. Reporting and using information extracted from these reports about medication errors and near misses are essential to improve patient safety and to avoid their recurrences (Koczmara, 2006(. Despite Reporting error is the most common method for Identifying error types, its frequency, consequences and prevent future mistakes, there is underreporting (Hogan et al., 2008). Underreporting medication errors is a worldwide problem.
In the USA, Reporting system missed 90% of adverse events (Hogan et al., 2008). In another study estimated underreporting of adverse drug events to range from 50%–96% annually (Leape, 1994). Reporting errors in hospitals occurring in at least one and maybe three out of ten errors (Huges and Blegen, 2008). In England, the rate of reporting simple error is 22%-39%, while more severe errors often go no reported (Leigh, 2006). In Saudi Arabia, Medication errors are under-reported (Alshaikh et al. 2013;