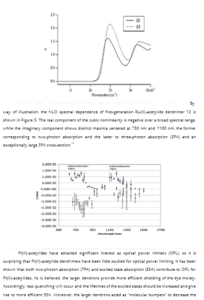
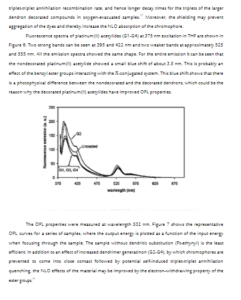
Syntheses and Nonlinear Optical (NLO) Properties of Ru(II) and Pt(II)
– Acetylide Dendrimers
Hana Mohammed Alanazi
Department of chemistry || faculty of science || King Abdulaziz university || Jeddah || KSA
تحضير ودراسة الخصائص الضوئية الغير خطية لمركبات أسيتيليد الروثينيوم والبلاتينيوم الشجرية
هناء محمد العنزي
كلية العلوم || جامعة الملك عبد العزيز || جدة || المملكة العربية السعودية
-
Introduction
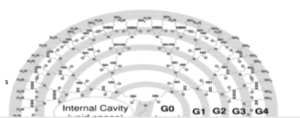
The dendritic polymer includes both dendrimers and hyper branched polymer. Dendrimers are a new class of polymeric materials, first discovered in the early 1980’s by Donald Tomalia and co-workers, these hyperbranched molecules were called dendrimers.1 At the same time, Newkome’s group independently reported synthesis of similar macromolecules.2 They called them arborols from the Latin word ‘arbor’ also meaning a tree. The term cascade molecule is also used, but ‘dendrimer’ is the best established one. Dendrimers are tree like in structure and appearance. A large number of branches during polymer synthesis lead to formation of macromolecule with many end groups. Out of highly branched polymers, dendrimers are perfectly branched uniform structure and hyper branched polymers are randomly branched. ‘Dendrimer’ is not a compound; it is only an architectural motif.
Generally accepted definition of dendrimer is a monodisperse macromolecule with perfectly branched regular structure and having at least one branched junction at each repeat unit that built around a small molecule or a linear polymer core. Dendrimers are a new class of polymeric belongings. Their chemistry is one of the most attractive and rapidly growing areas of modern chemistry.
Dendrimers are well-defined three-dimensional macrostructures. The pseudospherical shape of a dendrimer arises from its structure, which consists of an internal region (the core) which is connected to repeating units (grow through a variety of chemical reactions), constituting a radial branching pattern. These macromolecules tend to linearly increase in diameter and adopt a more globular shape with increasing dendrimer generation (Figure 1).3 Therefore, dendrimers have become an ideal delivery vehicle candidate for exploit study of the effects of polymer size, charge and composition on biologically relevant properties including lipid bilayer interactions, cytotoxicity, internalization, blood plasma retention time, biodistribution and filtration.4 There is a debate about the exact structure of dendrimers, in particular whether they are fully extended with maximum density at the surface or whether the end-groups fold back into a densely packed interior. Dendrimers of lower generations (0, 1, and 2) have highly asymmetric shape and possess more open structures as compared to higher generation dendrimers. As the chains growing from the core molecule become longer and more branched (in 4 and higher generations) dendrimers, adopting a globular structure. Dendrimers become densely packed as they extend out to the periphery, which forms a closed membrane-like structure. When a critical branched state is reached, dendrimers cannot grow because of a lack of space. This is called the ‘starburst effect’.5, 6