Ahmed Shawky Abdel- Sattar Mokhtar Mohammed Ibrahim Al Zawahiri Hussein Hassan Thabet
MUST university || Egypt
Khaled Abdul- Ghani Baraka
Menia university || Egypt
INTRODUCTION:
Cardiovascular diseases (CVD) or heart diseases are a class of diseases that involve the heart or blood vessels. Diseases under the heart disease umbrella include blood vessel diseases, such as coronary artery disease (CAD), heart rhythm problems (arrhythmias), heart infections and heart defects including congenital heart defects (1, 2, 3). Heart failure is a common clinical syndrome that represents the final stage of a range of different heart diseases (4, 5).
Clopidogrel is a second generation thienopyridine that is transformed in the liver to active form that is specially and irreversibly to platelets P2Y12 receptor, inhibiting ADP- mediated platelets activation and aggregation of note, most of the prodrug (around 85%) is hydrolyzed to inactive metabolites by esterase, leaving only 15% available for transformation to active metabolite two sequential oxidative reactions are necessary to form active metabolite, involving CYP1A2, CYP2B6, and CYP2C19, CYP2C9, CYP3A4, and CYP3A5, respectively (6, 7) dual antiplatelet therapy with clopidogrel and aspirin reduces cardiovascular death and ischemic complications in patients with ACS and those undergoing PCI(8).However, wide interindividual variability in platelet aggregation is commonly observed during dual antiplatelet therapy and some patients still experience thrombotic events (9, 10).
Cytochrome P450 (CYP450) is the generic name given to a large family of enzymes that metabolize most drugs and chemicals of toxicological importance. In humans, there exist 18 mammalian CYP450 families, 44 subfamilies, 57 putative functional enzymes(11, 12). The family members CYP1, CYP2, and CYP3 of CYP450 represent the top candidate genes in pharmacokinetics (13, 14). Four CYP2C genes have been identified in humans: CYP2C8, CYP2C9, CYP2C18, and CYP2C19. CYP2C19 enzyme is one of the hepatic cytochrome P450 enzymes that metabolize many important clinical drugs including antiulcer drug omeprazole, antiplatelet drug clopidogrel, anticonvulsant me phenytoinbertilsson, antimalarial drug proguanilthe anxiolytic drug diazepam, and certain antidepressants such as citalopram (15, 16).
There are 35 different variant alleles of CYP2C19(17). The CYP2C19*1 allele is the normal (wild- type) copy that has full enzymatic activity (18). The most common variant allele is named as CYP2C19*2 which result in truncated, nonfunctional protein (19). Another common allele is named as CYP2C19*3 which also creates a premature stop codon (20). Genetic polymorphisms of CYP2C19 are associated with impaired clopidogrel metabolism in healthy and in patients (21). Research on CYP2C19 enzyme among egyptional patients is rare. No previous study linked CYP2C19 with the outcome of drug metabolism. The present study is the first to determine cytochrome P450 2C19 polymorphism and to asses Clopidogrel management in patients with CAD in egyptional patients.
Platelet activation and coagulation do not typically occur in intact blood vessels. Rather, these processes are initiated in response to vascular injury or atherosclerotic plaque rupture, which also leads to vasoconstriction at the damaged site to attenuate blood loss. The endothelial cells of the compromised vessels subsequently secrete vonwill brand factor, collagen, and other tissue factors to the blood stream that adhere to and activate circulating platelets (22) activated platelets change shape.
To facilitate further adhesion, initiate the arachidonic acid pathway to produce thromboxane A2(TXA2) and excrete the contents of their granules, releasing ADP, serotonin, and other proteins. Appositive feedback mechanism promotes further vasoconstriction, addition platelet localization, activated platelet initiate the intrinsic, extrinsic coagulation pathways, which leads to thrombin- mediated conversion of fibrinogen to fibrin.
PATIENTS, MATRIAL, METHODS
Patients
Total 102 patients involved in descriptive cross- sectional study were enrolled from Eldoaa governmental hospital and Cairo health care specialized hospital between April 2017 and April 2018.The ethics committee of the misr university for science and technology approved the study and all patients provided written informed consent. Procedures were carried out in accordance with the approved guidelines.This study was designed to determine the frequency of CYP2C19 polymorphism in CHD patients undergoing PCI and the effect of this polymorphism on 1- year clinical outcomes. Patients were eligible for study enrollment in the cases where (1)ages were higher than 30 and (2) they were diagnosed with CHD using coronary angiography and successfully treated with PCI. Exclusion criteria were as follows (1) aspirin, clopidogrel and contrast agent allergy (2) severe diseases such as tumors and bleeding disorders, (3) hemodynamic instability (systolic blood pressure < 90mmhg and/or diastolic blood pressure <50mmhg) severe ventricular dysfunction (Left ventricular ejection fraction <40 %) and (4) severe liver and kidney dysfunction.
Matrials, Methods
PCI and clopidogrel treatment
PCI was performed in accordance with the standard of care. All patients were administrated 300mg clopidogrel and 40mg atorvastatin prior to operation, operated decided on the choice of anticoagulant and use of glycoprotein IIb/IIIa inhibitor (tirofiban, as well as the type of stent. After PCI patients were treated with 100mg/day aspirin and 75mg/day of clopidogrel for 1 year. We define the success of PCI as follows, (1) achievement of complete revascularization and residual stenosis of < 20% in target vessels of patients, (2) level 3 TIMI blood flow, (3) partial or complete remission of symptoms of myocardial ischemia and (4) no serious complications during hospitalization ( such as acute myocardial infarction, urgent target lesion revascularization, death).
Genotype analysis
CYP2C19 genotype was identified using clopidogrel sensitive gene detection kit(DNA- technology Company, Russia).DNA was extracted from whole blood, master mix is prepared, 3pcr tubes is prepared and each tube contain 20mcq from each specific prope, 10 mcq master mix, 20mcq mineral oil, 5mcq DNA all steps were performed in strict accordance to the DNA- technology genetic analyzer manual instructions. According to the CYP2C19*1, *2*3 genotypes, patients carrying *1/*1 were grouped as extensive metabolizers, *1/*2 or *1/*3 as intermediate metabolizers, and *2/*2, *2/*3, *3/*3 as poor metabolizers.
Clinical Endpoints
The clinical endpoints include the following MACE: cardiac death, myocardial infarction, and unstable angina cardiac deaths was defined as any death due to cardiovascular causes or not clearly attributable to non- cardiovascular causes myocardial infarction was defined as recent ischemic symptoms with ST- segmental elevation or depression and abnormally elevated troponin. Unstable angina was defined as resting onset, duration greater than or equal to 20 min or more severe and longer duration of angina than before and higher frequency of onset. Diagnosis was performed during hospitalization according to serum creatine kinase levels, electrocardiogram findings and other indicators.
Statistical analysis
Data was entered in a spreadsheet program. Discrete values were presented in numbers and percentages and inferences were made by chi square test of significance was used in order to compare proportions between two qualitative parameters. Binary logistic regression was used to predict the outcome of categorical variable based on one or more predictor variables the confidence interval was set to 95% and the margin of error accepted was set to 5%. Therefore, the p- value was considered significant was as following:
- P- value < 0.05 was considered significant
- P- value < 0.001 was considered as highly significant
- P- value> 0.05 was considered insignificant
Results:
Study population:
Total of 102 patients provided written informed consent based on inclusion criteria, all of them were enrolled for 1 year follow up with follow –up rate about 100 %, according to CYP2C19 genotype 50, 52 patients were grouped as normal metabolizer and abnormal metabolizers (IM, ULTRAMETABOLIZERS), from abnormal metabolizers, 17 patients were IM, 35 patients were ultra metabolizers
Distribution of the demographic data in the study groups.
From the results in Table (1) and Figures (1, 2 and 3) it could be noticed that the patients <60 years (55.88%) and ≥60 (44.12%) of age (years) were showed in Figure (1), also female (37.25%) and male (62.75%) of sex were showed in Figure (2), meanwhile rural (45.1%) and urban (54.9%) were reported in Figure (3) according to demographic data.
Table (1) Distribution of the demographic data in the study groups.
| demographic hic data | Total (n=102) |
| Age (years) | |
| <60 years | 57 (55.88%) |
| ≥60 years | 45 (44.12%) |
| Range [Mean±SD] | 38- 72 [55.78±7.05] |
| Sex | |
| Female | 38 (37.25%) |
| Male | 64 (62.75%) |
| Residence | |
| Rural | 46 (45.1%) |
| Urban | 56 (54.9%) |
Distribution of risk factors in the study groups.
Table (2)and Figure (4) showed that the DM, HTN and smoking as a risk factors were49 (48.04%), 56 (54.9%) and 40 (39.22%), respectively.
Table (2) Risk factors distribution of the study group.
| Risk factors | Total (n=102) |
| DM | 49 (48.04%) |
| HTN | 56 (54.9%) |
| Smoking | 40 (39.22%) |
Follow up MACE distribution of the study group
Table (3) and Figure (5) showed that the Non STEMI 1 (0.98%), Ischemic Dilated Cardiomyopathy 1 (0.98%), Myocardial Infarction 8 (7.84%), Unstable Angina 28 (27.45%) and Non 64 (62.75%), respectively, of follow up.
Table (3) Follow up MACE distribution of the study group.
| Follow up (MACE) | Total (n=102) |
| Non STEMI | 1 (0.98%) |
| Ischemic Dilated Cardiomyopathy | 1 (0.98%) |
| Myocardial Infarction | 8 (7.84%) |
| Unstable Angina | 28 (27.45%) |
| Non | 64 (62.75%) |
Genotyping distribution of the study group.
Table (4) and Figure (6) showed that the *1*1 (0.98%), *1/*1 (48.04%), *1/*17 (34.31%), *1/*2 (14.71%), *1/*3 (0.98%) and *2/*17 (0.98%) of genotyping.
Table (4) Genotyping distribution of the study group.
| Genotyping | Total (n=102) |
| *1*1 | 1 (0.98%) |
| *1/*1 | 49 (48.04%) |
| *1/*2 | 15 (14.71%) |
| *1/*3 | 1 (0.98%) |
| *1/*17 | 35 (34.31%) |
| *2/*17 | 1 (0.98%) |
Phenotyping distribution of the study group.
The present results in Table (5) and Figure (7) illustrated that the Abnormal metabolizer 52 (50.98%) and Normal metabolizer 50 (49.02%) of phenotyping. Meanwhile, Ultrametablizer andIntermediate metabolizer were 35(34.31%) and 17(16.67%), respectively.
Table (5) Phenotyping distribution of the study group.
| phenotyping | Total (n=102) |
| Abnormal metabolizer | 52 (50.98%) |
| Intermediate metabolizer | 17 (16.67%) |
| Ultrametablizer | 35 (34.31%) |
| Normal metabolizer | 50 (49.02%) |
Comparison between normal metabolizer and abnormal metabolizer according to demographic data.
From the results in Table (6) could be noticed that the statistically analysis was significantly difference between normal metabolizer and abnormal metabolizer according to age (years) and residenence.
Table (6) Comparison between normal metabolizer and abnormal metabolizer according to demographic data.
| Demographic Data | Normal metabolizer (n=50) |
Abnormal metabolizer (n=52) |
x2 | p- value |
| Age (years) | ||||
| <60 years | 41 (82.0%) | 16 (30.8%) | 27.136 | <0.001** |
| ≥60 years | 9 (18.0%) | 36 (69.2%) | ||
| Sex | ||||
| Female | 18 (36.0%) | 20 (38.5%) | 0.066 | 0.797 |
| Male | 32 (64.0%) | 32 (61.5%) | ||
| Residence | ||||
| Rural | 32 (64.0%) | 14 (26.9%) | 14.153 | <0.001** |
| Urban | 18 (36.0%) | 38 (73.1%) | ||
x2: Chi- square test; **p- value <0.001 HS
Comparison between normal metabolizer and abnormal metabolizer according to risk factors.
Table (7) and Figure (11) showed that highly statistically significantly analysis difference between normal metabolizer and abnormal metabolizer according to risk factors.
Table (7) Comparison between normal metabolizer and abnormal metabolizer according to risk factors.
| Normal metabolizer (n=50) |
Abnormal metabolizer (n=52) |
x2 | p- value |
| 16 (32.0%) | 33 (63.5%) | 10.108 | <0.001** |
| 18 (36.0%) | 38 (73.1%) | 14.153 | <0.001** |
| 8 (16.0%) | 32 (61.5%) | 22.176 | <0.001** |
x2: Chi- square test; **p- value <0.001 HS
Statistically significant difference between normal and abnormal metabolizer
Table (8) and Figure (12) showed that statistically significantly analysis difference between normal metabolizer and abnormal metabolizer according to Myocardial Infarction, Unstable Angina and non.
Table (8)This table shows highly statistically significant difference between normal metabolizer and abnormal metabolizer according to risk factors.
| Follow Up (MACE) | Normal metabolizer (n=50) |
Abnormal metabolizer (n=52) |
x2 | p- value |
| Non Stemi | 1 (2.0%) | 0 (0.0%) | 0.002 | 0.984 |
| Ischemic Dilated Cardiomyopathy | 1 (2.0%) | 0 (0.0%) | 0.002 | 0.984 |
| Myocardial Infarction | 1 (2.0%) | 7 (13.5%) | 4.201 | 0.047* |
| Unstable Angina | 8 (16.0%) | 20 (38.5%) | 5.397 | 0.020* |
| Non | 39 (78.0%) | 25 (48.1%) | 8.513 | 0.004* |
x2: Chi- square test; *p- value <0.05 S
Logistic regression of factors affecting normal and abnormal metabolizer.
From the results inTable (9) and Figure (13) it could be found that age (years), residence, HTN and smoking, have a significant effect on the normal metabolizer and abnormal metabolizer.
Table (9) Logistic regression of factors affecting normal metabolizer and abnormal metabolizer.
| factors | B | S.E. | Sig. | OR | 95% C.I | |
| Lower | Upper | |||||
| Age (years) | 0.082 | 0.041 | 0.045* | 1.086 | 1.002 | 1.177 |
| Residence | – 1.722 | 0.553 | 0.002* | 0.179 | 0.060 | 0.529 |
| DM | – 0.736 | 0.567 | 0.195 | 0.479 | 0.158 | 1.457 |
| HTN | – 1.249 | 0.553 | 0.024* | 0.287 | 0.097 | 0.848 |
| Smoking | – 2.085 | 0.605 | <0.001** | 0.124 | 0.038 | 0.407 |
OR: Odds Ratio; *p- value <0.05 S; **p- value <0.001 HS
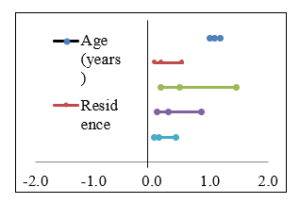
Fig. (1) Odds ratio affecting normal metabolizer and abnormal metabolizer.
Discussion
Cardiovascular diseases are globally the killer number one, representing 31% global deaths in 2014. Of these deaths estimated 7.4 million were due to coronary heart disease(23, 24). The current standard management for CAD is a dual antiplatelet therapy (aspirin and clopidogrel), in addition to coronary reperfusion or revascularization(25, 26).Knowledge of the individual’s genotype can be used to optimize clopidogrel antiplatelet therapy(27). The pharmacodynamics response to clopidogrel varies widely from subject to subject, and about 25% of patients treated with standard clopidogrel doses display low inhibition of ADP- induced platelet aggregation(28).this poor response to clopidogrel is associated with an increased risk of recurrent ischemic events(29).
Genetic polymorphism in CYP2C19, an enzyme required for clopidogrel bio activation have been shown to be associated with clopidogrel antiplatelet effectiveness, and represents a risk factor for recurrent ischemic cardiac events (30).
To the best of our knowledge, this is the first study in Egyptian population reporting the impact of CYP2C19 loss of function alleles, CYP2C19*2 and CYP2C19*3, in association with recurrent ischemic attacks in patients managed with clopidogrel after PCI, and this second study in Egyptian population reporting frequency of CYP2C19 genetic polymorphism.
The importance of this study related to fact that an understanding of distribution of SNPs is crucial for the future application of pharmacogenomics to different population groups. Such applications have recently been performed in warfare in therapy (31)in our study, the frequency of CYP2C19 *1, *2 and *3, *17 was 48%, 17%, 1%, 34% respectively, allelic frequency of CYP2C19*2 is higher than reported in Iraqi population (15.2%) (32), Saudi Arabia(15%)(33), Jordanian(16%)(34), Lebanese (13.4%)(35), Tunisian region (11.5%) (36). German (15.2%) (37), Belgian(9.1%) (38)Ethiopians (14%) (39), and lower than reported in Japanese (27.4%)(40), Chinese (45.5%) (41), Koreans(20.9%)(42). Malaysians (28%) (43).
In first study in Egyptians population( 44)the allelic frequency (12%) is lower than reported in our study. allelic frequency of CYP2C19 *3 is lower than reported in Lebanese (3%) (35), Ethiopians (3%) (38)
Koreans (11.7%)(41)and Japanese (10.8%) (40) and higher than reported in Iraqi population (0.2%)(32), Saudi Arabian population(0.00%)(33), Italians population(0%)(45), Russians (0.3%) (46) and also, the same as Tanzanians population (1%) (47).Allelic frequency of CYP2C19*17 is higher than frequency reported from Saudi Arabian (25.7%), Iraqi population (19.4%), Greece population(19.6%) (48) and African Americans (21.0%)(49).
The current study focused also on determining whether CYP2C19 genetic variants modulate clopidogrel- mediated antiplatelet effects and risk of MACE in patients undergoing PCI after 1 year follow up our main finding were as follow:1- there is difference between abnormal metabolizer(IM, ULRAMETABOLIZER) and normal metabolizer in MACE(p- value 0.047 for MI, and p- value 0.020 for unstable angina), according to logistic regression of factors affecting normal metabolizer and abnormal metabolizer, there is significant effect of age, residence, HTN, smoking on abnormal metabolizer and normal metabolizer.
Several studies have consistently reported that the CYP2C19*2 polymorphism is associated with lower antiplatelet response and increase the rate of recurrent cardiovascular events in Caucasian, Korean, and chines patients (50, 51, 52 53).moreover, the CYP2C19*3 variant is associated with clopidogrel –mediated antiplatelet effects in Asia since the incidence
Conclusion: Asignificant relation between CYP2C19 polymorphism and clinical outcome of ischemic heart disease patients taking clopidogreal after PCI especially smoking, hypertensive patients but meta- analysis study is required to confirm this result.
References
- Gaziano, T. (2005). Cardiovascular Disease in the Developing World and Its Cost- Effective Management. Circulation, 112, 3547- 3553
- CDC (2009). Centers for Disease Control and Prevention CDC. (2009). Heart Disease. Coronary Artery Disease. Retrieved in December 2015.
- Mozaffarian, D., Benjamin, E.J., Go, A.S., Arnett, D.K., Blaha, M.J., Cushman, M., & Howard, V.J. (2015). Heart Disease and Stroke Statistics- 2016 Update. Circulation. Retrieved in September 2015 from:
https://circ.ahajournals.org/content/early/2014/12/18/CIR.0000000000000152.full.pdf
- Anderson, J.L., Adams, C.D., Antman, E.M., Bridges, C.R., Califf, R.M., Casey, D.E., &Chavey, W.E. (2007). Guidelines for the management of patients with unstable angina/non–ST- elevation myocardial infarction. Journal of American College of Cardiology, 50(7): 150- 157.
- Kazui M, Nishiya Y, Ishizuka T. (2010) Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug MetabDispos 38:92–99.
- Sadeghi, R., Adnani, N., Erfanifar, A., Gachkar, L., &Maghsoomi, Z. (2013). Premature Coronary Heart Disease and Traditional Risk Factors- Can We DoBetter?. International Cardivascular Research Journal, 7 (2): 46- 50.
- Stewart, R., Held, C., Brown, R., Vedin, O., Hagstrom, E., Lonn, E., Armstrong, P., Granger, C.B., Hochman, J., Davies, R., Soffer, J., Wallentin, L. and Lane, H. (2013). Physical activity in patients with stable coronary heart disease: an international perspective. European Heart Journal, 34(42), 3286- 3293
- Gurbel, P.A., Bliden, K.P., Hiatt, B.L. and O’Connor, C.M. (2003). Clopidogrel for coronary stenting response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation, 107(23), 2908- 2913.
- Müller I, Besta F, Schulz C, Massberg S, Schönig A, Gawaz M. (2003). Prevalence of clopidogrel non- responders among patients with stable angina pectoris scheduled for elective coronary stent placement. ThrombHaemost. 2003;89(5):783–7.
- Ingelman- Sundberg, M. (2005). The human genome project and novel aspects of cytochrome P450 research. Toxicology and Applied Pharmacology 207, 52- 56.
- Jensen, L.O., Maeng M., Kaltoft, A., Thayssen, P., Hansen H.H., Bottcher M., Lassen J.F., Krussel L.R., Rasmussen K., & Hansen K.N. (2007). Stent thrombosis, myocardial infarction, and death after drug- eluting and bare- metal stent coronary interventions. Journal of the American college of cardiology, 50(5), 463- 470.
- Yang, X., Zhang, B., Molony, G., Chudin, E., Hao, K., Zhu, J., Gaedigk, A., Suver, C., Zhong, H., Leeder, S., Guengerich, F.P., Stephen, C., strom., Schuetz E., Thomas H., Rushmore., Roger G, Ulrich., Slatter G., Schadt E.E., Kasarskis, A. and Lum, P.Y (2010). Systematic genetic and genomic analysis of cytochrome P450 enzyme activities in human liver. Genome Research, 20 (8): 1020- 1036.
- Knights, K.M., Rowland, A. and Miners J.O. (2013). Renal drug metabolism in humans: the potential for drug–endobiotic interactions involving cytochrome P450 (CYP) and UDP glucuronosyl transferase (UGT). British Journal ofClinical Pharmacology, 76 (4), 587- 602.
- Flachsbart, f., Ufer, M., Kleindorp, R., Nikolaus, S., Schreiber, S., and Nebel, A. (2011). genetic variation in the CYP2C monooxygenase enzyme subfamily shows no association with longevity in a german population. Journal of Gerontology: Biological Sciences 66A, 11, 1186- 1191.
- Lee, S.J. (2013). Clinical application of CYP2C19 pharmacogenetics toward more personalized medicine. Frontiers in Genetics, 3, 1- 13.
- OMIM, Online Mendelian Inheritance in Man (OMIM). (2016). Cytochrome p450, subfamily 2C, polypeptide 19; CYP2C19. Retrieved in January 2016. http://www.cypalleles.ki.se/cyp2c19.htm
- Kubica, A., Kozinsk, M., Grzesk, G., Fabiszak, T., Navarese, E. and Goch, A. (2011). Genetic determinants of platelet response to clopidogrel. Journal of Thrombosis and Thrombolysis, 32 (4), 459- 466.
- Mega JL, Close SL, Wiviott SD. (2009). Cytochrome P- 450 polymorphisms and response to Clopidogrel. N Engl J Med 360: 354–362.
- Nakamoto, K., Kidd, J., Jenison, R., Klaassen, C., Wan J., Kidd A. and Zhong, X. (2007). Genotyping and haplotyping of CYP2C19 functional alleles on thin- film biosensor chips. Pharmacogenetics and Genomics, 17(2), 103- 114.-
- Sangkuhl, K., Klein, T.E. and Altman, R.B. (2010). Clopidogrel pathway. Pharmacogenet Genomics, 20 (7), 463- 465.
- World Health Organization (2015). Cardiovascular diseases (CVDs): fact sheet. Retrieved in January 2015.http://www.who.int/mediacentre/factsheets/fs317/en/.
- Sakakura, K., Nakano, M., Otsuka, F., Ladich, E., Kolodgie, F., and Virmani R. (2013). Pathophysiology of Atherosclerosis Plaque Progression. Heart, Lung and Circulation, 22(6), 399- 411
- WHO, World Health Organization (2015). Cardiovascular diseases (CVDs): fact sheet. Retrieved in January 2015.
- Mathew, V., Gersh, B.J., Williams, B.A., Laskey, W.K., Willerson, J.T., Tilbury, R.T., Davis, B. and Holmes, D.R. (2004). Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era A report from the prevention of restenosis with tranilast and its outcomes (PRESTO) trial. Circulation, 109(4), 476- 480.
- Ferreiro, J.L. and Angiolillo, D.J. (2012). New directions in antiplatelet therapy. Circulation: Cardiovascular Interventions, 5(3), 433- 445.
- W. (2015).Corporate Social Responsibility Practice from 1800–1914: Past Initiatives and Current Debates. Business Ethics Quarterly / Volume 25 / Issue 01 / January 2015, pp 125- 141
- Hagymási K, Müllner K, Herszényi L, Tulassay Z (2011) Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics 12: 873- 888.
- Matetzky, S., Shenkman, B., Guetta, V., Shechter, M., Beinart, R., Goldenberg, I., Novikov, I., Pres, H., Savion, N., Varon, D. andHod, H. (2004). Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation, 109(25): 3171- 3175.
- Hulot, J.S., Bura, A., Villard, E., Azizi, M., Remones, V., Goyenvalle, C., Aiach M., Lechat, P. and Gaussem, P. (2006). Cytochrome P450 2C19 loss- of- function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood, 108(7), 2244- 2247.
- Nakata K, Takami A, Espinoza JL. (2013). The recipient CXCL10 + 1642C>G variation predicts survival outcomes after HLA fully matched unrelated bone marrow transplantation. Clin Immunol. 2013;146(2):104- 111.
- Ross GW, Petrovitch H, White LR, Masaki KH, Li CY, Curb JD, Yano K, Rodriguez BL, Foley DJ, Blanchette PL, HavlikR.(1999). Characterization of risk factors for vascular dementia: the Honolulu- Asia Aging Study. 1999;53:337–343.
- Hussein Ali Sahib, BassimIrhiem Mohammed, and Ban A. Abdul- Majid(2015). Genetic polymorphism of cyp2c19 in a sample of Iraqi population. International Journal of Pharmacy and Biological Sciences, 5 (4): 54- 60
- Saeed, L.H. and Mayet, A.Y. (2013). Genotype- Phenotype analysis of CYP2C19 in healthy Saudi individuals and its potential clinical implication in drug International journal of medical science, 10(11), 1497- 502.
- Zalloum I, Hakooz N and Arafat, T.(2012).Genetic polymorphism of CYP2C19 in a Jordanian population: influence of allele frequencies of CYP2C19*1 and CYP2C19*2 on the pharmacokinetic profile of lansoprazole. Mol Biol Rep., 39(4):4195- 4200.
- Djaffar Jureidini, I., Chamseddine, N., Keleshian, S. (2011). Prevalence of CYP2C19 polymorphisms in the Lebanese population Biol. Rep. 38 (8), 5449–5452.
- Abid, L., Laroussi, L., Bahloul, A., Siala, A., Abdelhédi, R., Kharrat, N., Hentati, M. and Kammoun, S. (2013). Impact of cytochrome P450 2C19* 2 polymorphism on the clinical cardiovascular events after stent implantation in patients receiving clopidogrel of a southern Tunisian region. World Journal of Cardiovascular Diseases, 3 (1), 4- 10.
- Geisler T, Schaeffeler E, Dippon J, et al (2008). CYP2C19 and nongenetic factors predict poor responsiveness to clopidogrel loading dose after coronary stent implantation. Pharmacogenomics; 9: 1251- 1259
- Allabi AC, Gala JL, Desager JP. (2003). Genetic polymorphisms of CYP2C9 and CYP2C19 in the Beninese and Belgian populations. Br J Clin Pharmacol, 56(6):653- 657.
- Persson I, Aklillu E, Rodrigues F. (1996). S- mephenytoin hydroxylation phenotype and CYP2C19 genotype among Ethiopians. Pharmacogenetics, 6(6):521- 526, (1996)
- Sugimoto K, Lino T, Yamazalci H, et al. Limited frequency of the CYP2C19*17 allele and its minor role in a Japanese population. Br J Clin Pharmacol, 65: 437- 439, (2008)
- Yamada S, Onda M, Kato S, et al. Genetic differences in CYP2C19 single nucleotide polymorphisms among four Asian populations. J Gastroenterol, 36(10):669- 672, (2001)
- Roh HK, Dahl ML, Tybring G, et al. CYP2C19 genotype and phenotype determined by omeprazole in a Korean population. Pharmacogenetics, 6(6):547- 551, (1996)
- Pang YS, Wong LP, Lee TC, et al. Genetic polymorphism of cytochrome P450 2C19 in healthy Malaysian subjects. Br J Clin Pharmacol, 58(3):332- 335, ( 2004)
- Hamdy SI, Hiratsuka M, Narahara K, et al. Allele and genotype frequencies of polymorphic cytochromes P450 (CYP2C9, CYP2C19, CYP2E1) and dihydropyrimidine dehydrogenase (DPYD) in the Egyptian population. Br J Clin Pharmacol, 53:596- 603, (2002)
- Scordo, M. G., Caputi, A. P., D’Arrigo, C. (2004). Allele and genotype frequencies of CYP2C9, CYP2C19 and CYP2D6 in an Italian population. Pharmacol Res 50:195–200.
- Gaikovitch, E. A., Cascorbi, I., Mrozikiewicz, P. M., Brockmoller, J., Frotschl, R., Kopke, K., Gerloff, T., Chernov, J. N., Roots, I. (2003). Polymorphisms of drug- metabolizing enzymes CYP2C9, CYP2C19, CYP2D6, CYP1A1, NAT2 and of P- glycoprotein in a Russian population. Eur. J. Clin. Pharmacol. 59, 303- 312.
- Herrlin K, Massele AY, Jande M, Alm C, Tybring G, Abdi YA, Wennerholm A, Johansson I, Dahl ML, Bertilsson L, Gustafsson LL (1998) Bantu Tanzanians have a decreased capacity to metabolize omeprazole and mephenytoin in relation to their CYP2C19 genotype. Clin PharmacolTher. 64:391–401
- Ragia, G.; Arvanitidis, K.I.; Tavridou, A.; Manolopoulos, V.G.(2009). Need for reassessment of reported CYP2C19 allele frequencies in various populations in view of CYP2C19*17 discovery: The case of Greece. Pharmacogenomics, 2009, 10, 43–49.
- Xie HG, Kim RB, Stein CM. (1999). Genetic polymorphism of (S)- mephenytoin 4′- hydroxylation in populations of African descent. Br J Clin Pharmacol, 48(3):402- 408, (1999)
- Brandt, J.T., Clos, e S.L., Iturria, S.J., Payne, C.D., Farid, N.A., Ernest, C.S., Lachno, D.R., Salazar, D. and Winters, K.J. (2007). Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamics response to clopidogrel but not prasugrel. Journal of Thrombosis Hemostasis, 5(12), 2429- 2436.
- Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. (2009). Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. 2009;302:849–857. [PMC free article].
- Hochholzer W, Trenk D, Fromm MF. (2010).Impact of cytochrome P450 2C19 loss‐of‐function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J. Am. Coll. Cardiol. 2010;55:2427–2434.
- Oh IY, Park KW, Kang SH. (2012). Association of cytochrome P450 2C19*2 polymorphism with clopidogrel response variability and cardiovascular events in Koreans treated with drug- eluting stents. Heart 98, 139- 44 (2012).
تعداد التحور الجيني لجين السيتوكروم ب450 وتأثيره على النتائج السريرية في مرضي نقص تروية القلب الذين يتناولون عقار كلوبيدوجريل بعد التدخل وتركيب دعامات بالقسطرة العلاجية