Silver nanoparticles (AgNPs) from plant extracts
Samiyah Saeed Al-Zahrani
Faculty of Science || King Abdulaziz University || Jeddah || KSA
جسيمات الفضة متناهية الصغر من مستخلصات النبات
سامية سعيد الزهراني
كلية العلوم || جامعة الملك عبد العزيز || جدة || المملكة العربية السعودية
1.Introduction
Toxigenic fungi invade food products and produce health hazard mycotoxins as secondary metabolites in infected plants or in stored products, such as Penicillium, Fusarium Alternaria and Aspergillus spices which considered as the utmost significant producers of mycotoxins. The toxigenicity of these fungi threatening the lives of humans and animals worldwide. [1][2][3][4]. Since these fungi reported as critical responsible for the illness of human and his farm animals in world trade, the control of toxin producers is a matter of importance. Anti-fungal agent’s approaches have been exercised years ago to remove contaminant fungi with excluding damage to the host [5]. Thus, the concern in resistance of fungi and health-threatening exposure to chemical fungicides revolutionizing an economic attention to develop enhanced alternative antifungal agents. In this aspect, latest studies set out to eliminate this issue by creating a novel control of contaminant fungi with the aid of metal nanoparticles due their antimicrobial efficiency [6,7,8]. Today, nanotechnology has framed the premise of various applications and revealed the great efficiency of metal nanoparticles as antimicrobial agents likewise their applications in medicine and pharmaceuticals field [9][10]. Latest studies revealed that amid the metal nanoparticles silver nanoparticles (AgNPs) ideally hold huge assurance as antifungal agents towards fungal species as Aspergillus fumigatus [11,12] even towards fungal species A. flavus and, A. niger [12]. Exhaustive approaches have been generated to synthesis silver nanoparticles (AgNPs) from biological sources such as plants extracts and microorganisms. Afterwards, research scientists have raised concentration on plant capability to synthesis AgNPs. Green synthesis of nanoparticles, a term given to synthesis of (NPs) by biologically active composites of plant sources. By reason of plant’s rich wellspring of vast bioactive compounds such as, flavonoids, tannins, terpenoids, and alkaloids which may enhance the synthesis of AgNPs with the cost-less and eco-friendly method [13]. Nearly about 50 plant origin extracts confirmed the capability of synthesis AgNPs biologically. Besides, the AgNPs synthesize from plant extracts parts (leave, roots, stems, and seeds) were used as a part of conventional antimicrobials which can be beneficial assets for new antifungal agents [14]. Thus, this review was aimed one hand to summarize the capability of plant extracts in biosynthesis AgNPs. On the other hand, review the antifungal efficiency of the biosynthesized AgNPs from plant extracts specially against toxigenic fungi.
2. Mycotoxins
Toxigenic fungi known as a group of filamentous fungi occurring in food that are responsible for food deterioration and mycotoxins production. The World Health Organization (WHO) has identified mycotoxins as food-borne diseases of contaminant food and feed with fungi [15]. These mycotoxins are secondary metabolites can cause a variety of illness to humans and animals following consumption of contaminated food. Mycotoxins mainly produced by filamentous fungi mycelia of some fungal strains and server no significant function in the fungal physiological process [16,17]. Consumption of these mycotoxins can cause powerful toxic effects known as mycotoxicosis. St. Anthony’s Fire was the first mycotoxicosis that has been documented in the Middle Ages in France which called ergotism by the l850s. Ergotism was indicated after consumption of rye contaminated by Claviceps purpurea sclerotia [18]. Later, in 1948, trichothecene mycotoxin have been isolated for the first time by Freeman and Morrison from Trichothecium roseum which was suspected to be the etiological agent caused alimentary toxic aleukia in Russia during the world war II [19]. During the late 1950s, a mycotoxin called aflatoxin have been isolated from the mold Aspergillus flavus which increased the interest in mycotoxins and gave rise to people’s health guard by establishing limits of mycotoxins in food and feed by many countries [18]. Nowadays, almost four hundred mycotoxins have been isolated and chemically diagnosed. Nevertheless, research has been heavily focused on those causing critical harm to humans, animals, and crops [20]. Aflatoxins are the significant mycotoxins produced by Aspergillus species including four types known as aflatoxin B1, B2, G1, G2 which named for the color of their fluorescence under ultraviolet light, and their relative position on TLC plates [21]. Aflatoxins are produced by some strains of Aspergillus flavus and A. parasiticus. Theses molds found in field postharvest when moisture is present, allowing for the mold growth [ 22, 23]. Additionally, extensive researches have examined aflatoxins role in contamination of various commodities in stores such as corn, peanuts, cottonseed and almond [24 , 25]. The exposure to mycotoxins may occur at all levels of the food chain by consumption of contaminated plant materials products or from consumption of animals carrying mycotoxins in their milk or meat like aflatoxin M which found in milk, meat, or other products of animal origin [26]. It has been estimated that approximately 4.5 billion of the whole world’s population is exposed to aflatoxins [27]. While, ochratoxins poses a health hazard to human and animal’s health by causing nephrotoxic effects on all mammalian species [28]. Also, ochratoxins been known for its teratogenic, immunosuppressive and carcinogenic properties [29,30]. Furthermore, it may produce by some Penicillium species like Penicillium verrucosum which is the principal producer of ochratoxins and some Aspergilli species such as A. ochraceus and A. carbonarius [31].
2.1 Important toxigenic Aspergillus species
The genus Aspergillus is one of the most important filamentous fungal genera which form large mycotoxin producers. A. flavus which have a powdery colony with yellow-green spores containing degradative enzymes in the mycelium which make it capable of breaking down complex nutrients in plant materials [32]. Besides its capability to produces aflatoxins B1, B2, M1, and gliotoxin. As well A. parasiticus that known to produce aflatoxins, it is closely related to A. flavus. Nevertheless, A. parasiticus produces aflatoxins B1, B2, G1, and G2, unlike A. flavus which produces only aflatoxin B1. While A. niger produces gliotoxin, which founded in humans and mice serum that diagnosed with aspergillosis. In addition, A. niger may produce ochratoxins A, fumonisin B2, B4 and some other toxins [33].
Furthermore, A. ochraceus is mostly responsible for the production of ochratoxin A and other toxins it might produce as penicillic acid, xanthomegnin, and viomellein. Due to the significant threat of mycotoxins as aflatoxins (B1, B2, G1, and G2) produced by A. flavus and A. parasiticus and ochratoxins produced by A. ochraceus and A. niger to humans and domestic animals worldwide. This review aims to find an effective way to inhibit the growth of toxigenic fungi using AgNPs synthesized by plant extracts since prevention of their growth could eliminate their toxins. As AgNPs may be considered as useful candidates to eliminate aflatoxin contamination in food and feedstuffs [34].
There are various approaches used to control fungal growth thus eliminate mycotoxin biosynthesis in crops by using chemical processing, food preservatives, physical and biological treatments [34]. These methods need subtle instrumentation and valuable chemicals. Chemical management of fungi and mycotoxins make diverse sorts of environmental pollution peril and disturb the stability of the environment [35]. Using of plant substances inform of plant extract or its essential oils is accepted to be less harmful and provides a chance to avoid artificial chemical preservatives and chemical fungicide’s hazardous risks [36].
3. Plant extracts as natural antifungal agents
A wide range of antifungal agents are used in combating biodeterioration of food and food products. Carlile and Watkinson, 1996 clarified that every antifungal agent has its chemical nature, properties and its mode of action [37]. Antifungal compounds may be lethal to microorganisms or they may simply inhibit the production of metabolites such as mycotoxins. But certain number of studies showed that the use of sub-lethal concentration could favour the production of the toxins [38]. Plants, herbs, and spices as well as their essential oils, have a wellspring of numerous constituents that are known to inhibit various metabolic activities of bacteria, and fungi [36]. As a rule, plants with antimicrobial activity contains a wide range of polyphenolic compounds, terpenes, aldehydes, acids and flavonoids [13]. Plants extracts assume a crucial part in the improvement and progression of the antimicrobial agent which have framed the premise of numerous applications against a wide range of microbes, especially against fungi [13]. Further, plant extracts are secure and effective considering their antifungal properties which have been reported of several plant species by a large number of earlier workers over the years. The effectiveness of several plant active compounds on toxigenic molds indicates its possible exercise as fungicides to minimize mycotoxins hazardous exposure as well. Many reviews have demonstrated the inhibitory effect of plant extracts against fungi. Among several plants which have been studied for their antimicrobial properties, clove has received the most comprehensive studies [39]. As indicated, whole clove inhibited the growth of Aspergillus flavus and Penicillium citrinum and their mycotoxins in vivo and in vitro study [40, 41]. While many researchers reported the inhibitory effect of the clove oil with main component eugenol against Aspergillus sp. growth and aflatoxin B1 production [42, 43]. As well as the essential oils of several plants which showed noticeable antifungal properties such as the essential oils of Cinnamomum jensenianum, Ocimum sanctum, and Zataria multiflora have been efficacious on Aspergillus flavus growth and the amount of aflatoxin B1 produced [44] [45] [46]. The efficiency of neem extracts inhibiting the production of aflatoxins (B and G) in the mycelia has been reported [47]. While methanolic extracts of Agave asperrima and Agave striata reduced aflatoxin synthesis in A. flavus and A. parasiticus [48]. Several authors have confirmed that 100 plant species out of 280 have been examined for antifungal effect against toxic species of Aspergilli, have been effective in inhibiting the growth of fungi or inhibition of toxin production [49]. Many researches has been done in this area during recent years testing plant extracts and their essential oils [50,51,52,53,54]. Hence some plant components are showing an unusual antimicrobial activity protecting food and feed from toxigenic molds [55]. The most relevant studies reviewed were subsequently conducted by analyzing the active substances in plants essential oils that inhibited the growth of toxigenic fungi and their toxins [56]. As well plants component and plant products such as piperine (alkaloid extracted from piper species), lutein, xanthrophyll, and carotenoids which found naturally in some fruit and vegetables that markedly suppressed the toxicity and mutagenicity of aflatoxin B1[57,58,59]. The basic problem must be taken into account is the contact with plant extract or essential oils may enhance the fungal production of toxins. Thus, it is important to study each individual case since some plants such as the herbal plant Chromolaena odorata which considered to be potent against insects have induced toxigenic Aspergillus flavus and toxin production by making an excellent substrate for growth of storage fungi [60, 61]. Also, Khaya senegalensis bark used as insecticide have significantly increased the aflatoxin production in maize [62].
Nevertheless, caution must be applied, because some plant materials are a natural medium for toxigenic fungi and may make the situation worse. When inhibiting partial growth, we cannot be sure of toxin inhibition because in this case, the antifungal activity may stimulate the secretion of toxins and secondary metabolites in response to stress [63].
4. Nanotechnology
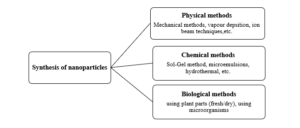
Nanotechnology is defined as the technology that uses nanomaterials in their design and applications (1-100) nm in size and having novel chemical and physical properties [64]. It is also the most promising field generating a new application in medicine [65]. Among all Nanometals, silver nanoparticles have been used since the 1880s [66]. Because silver has broad-spectrum antimicrobial activity against wide range of microorganisms which may induce much less resistance than alternative antibiotics [67]. Nanotechnology is fast-growing with nanoparticles produced and utilized in a wide range of pharmaceutical and commercial products worldwide. This environmentally friendly synthesis method of silver nanoparticles to using plant extracts is conventional to chemical synthesis and can potentially be used in various areas such as food, cosmetics, and medical applications [68]. Physically and chemically techniques for the production of metallic nanoparticles have been presented by the scientists in numerous research (Figure1). But, these synthetic methods involving different chemicals are expensive and may lead to the presence of toxic chemical tangled on the surface of nanoparticles, which may have adversarial effects in various biological and biomedical applications [69].
The growth of green biosynthesis methods of nanoparticles became an essential branch of nanotechnology in the 21st century [70]. The first report of the plant used in the synthesis of nanoparticles is credited to Medicago sativa which is capable to synthesis silver and gold nanoparticles [71]. Since then, enormous attention has been given to plant as a source of nanoparticles synthesis. Among all noble metal (NPs), silver nanoparticles had grown unlimited attention due to their exceptional properties such as chemical stability, excellent conductivity, catalytic and most important antimicrobial activity [72] and possess anti-fungal activity [73].
4.1 Silver nanoparticles (AgNPs)
In particular, silver nanoparticles (AgNPs) show good antimicrobial properties due to their large ratio of surface area to volume; these properties are used as bactericide on burn wounds, fillers in dental cavities to prevent infection, thin coats on medical devices to prevent microbial biofilm formation, in air and water purification systems, in wastewater treatment plants, and in food processing for controlling microbial contamination [74, 75]. Also, silver is known as being nontoxic and harmless to the human body at lower concentrations, contrasting other metal nanoparticles [76]. Lea,1889 had synthesized AgNPs for the first time. Then, several techniques for synthesis silver nanoparticles with various coating agents and diameters had been advanced and used commercially and therapeutically [75]. Recently, Nano-biotechnology has large interests to advance a new approach to test new drug formulations based on biosynthesized silver nanoparticles with different biological potential and physicochemical properties [77].
Figure (1( Different methods of nanoparticles synthesis
4.1.2 Antimicrobial properties of Silver nanoparticles
Silver nanoparticles have physical properties that are distinctive from silver ions and bulk material; their biological activity is greatly higher due to the high surface area to volume ratio that makes them promising antimicrobial against all types of pathogenic microorganisms [78, 79].The antibacterial effect silver nanoparticles have been represented against bacteria such as Escherichia coli, Vibrio cholera, Pseudomonas aeruginosa, and Salmonella typhi [80]. Also, in a study examined the antibacterial potential of silver nanoparticles on the pathogenic bacteria strains P. aeruginosa, resistant E. coli, and Streptococcus pyogenes [81]. Biosynthetic AgNPs showed high antibacterial activity against biofilm-forming S. epidermidis strain [82] and against E. coli [83]. These silver nanoparticles could exhibit inhibition to bacterial growth immediately when they contact them, by killing bacteria [84]. While AgNPs antimicrobial efficiency against bacteria has been studied more than against fungi. Consequently, bioactivity mechanism of AgNPs against fungal pathogens has not been indicated [85]. In addition to antibacterial activity, some authors reported the significant antifungal activity of AgNPs against Candida species and dermatophytes species Trichophyton mentagrophytes [86, 87] and against different fungal species like Dothiorella sarmentorum [88]. Whereas antifungal activities of AgNPs against different kinds of fungus have not been reported as much as antimicrobial activities of them by researchers [89]. It was reported that AgNPs could inhibit fungi in low concentrations and those levels had no toxic effect on human cells [90]. In a recent study, AgNPs showed inhibiting AFB1 production by A. parasiticus in addition to the ability for active fungal growth inhibition [91]. In another study, it showed antifungal activity against spoilage fungal isolates A. flavus and A. ochraceus [92].
4.5 Biosynthesis of silver nanoparticles using microorganisms
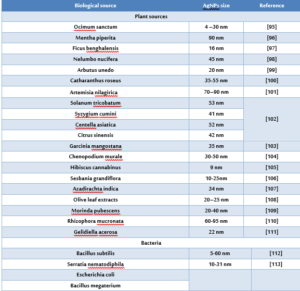
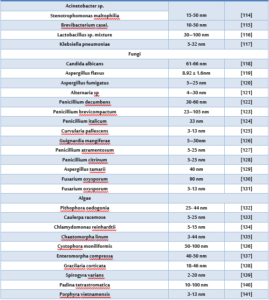
Nanoparticles prepared by different chemical and physical methods and biological methods. The physical and chemical methods are cost intensive and the use of hazardous chemicals during the processes limits their use in clinical applications. While the biological synthesis technique is an environmentally friendly procedure to usual chemical synthesis and can possibly be used in many areas such as food, cosmetics, and medical applications. Microorganisms (such as bacteria, fungi, yeast) have been used as potential nanofactories and as alternatives to the conventional routes to prepare metal nanoparticles [93, 94]. There are numerous studies which concerns on the ability of various fungi to biosynthesis AgNPs (Table1).
Table (1) Biosynthesis of silver nanoparticles using biological materials
4.6 Biosynthesis of silver nanoparticles using plant extracts
Plant-mediated synthesis or green synthesis of AgNPs is an economical way, non-hazardous and there is no need for chemical reducing and capping agents. Because of that numerous studies by researchers established to evaluate of their microbial activity. Moreover, they have huge applications in molecular biology and medicine (Figure 2).
A new process for producing AgNPs demonstrated by the reduction of AgNO3 in aqueous solutions with neem (Azadirachta indica) leaf broth [142]. Later, different green process for the preparation of AgNPs has been developed by using Eucalyptus citriodora and Ficus bengalensis fresh leaf extract along with AgNO3 in aqueous solution and without reducing or stabilizing agents at room temperature for 25 minutes. Reduction and stabilization of Ag+ ions were enhanced by functional groups present in the polysaccharide constituents in leaf extract [143].
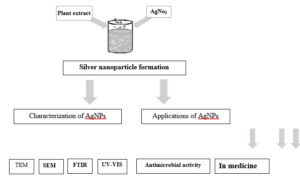
Figure (2) Methodology of Synthesis of silver nanoparticles using plant extract, characterization and application.
Recently, Silver nanoparticles have been biosynthesized by using leaf extracts of the plant such as Aloe vera leaf extract [144], Ricinus Communis leaves extract [145] leaf extracts of Piper nigrum [146] and Ocimum sanctum leaf extract [147]. Also, AgNPs have been biosynthesized by using root extracts of the plant such as Zingiber officinale root extract [148], And by using bark extracts such as Afzelia quanzensis bark extract [149]. AgNPs were prepared by biological reduction of AgNO3 as a precursor using Mentha piperita (Lamiaceae) leaf extract in ambient conditions, most probably because of the presence of phytochemicals in the extract, thereby reducing Ag+ into Ag0 [150]. In addition to using fruits extracts such as Momordica charantia fruit extract [151] and Prunus armeniaca fruit extract [152]. Although, seeds such as extracts of Sinapis arvensis seeds [153], Cydonia oblong seed [154] and seed extracts of Nyctanthes arbor-tristis [155]. A simple green and cost-effective approach for the synthesis of Ag-NPs with an average size of 12 nm using garlic clove extract as a reducing/stabilizing agent in aqueous solution was reported [156]. This was attributed to the presence of antioxidants within garlic extract and their decisive role in reducing Ag+ to Ag0. While the synthesis of AgNPs using banana peel extract as a reductant and AgNO3 as a precursor in an aqueous medium under various conditions was demonstrated [157]. Reduction of the nominated metal ions to nanoparticles as well as stabilization of these nanoparticles might be facilitated by the presence of reducing sugars, terpenoids, and flavanone constituents in the broth. Silver nanoparticles synthesized using plant extract like Solanus torvum [158], Argemone Mexicana [159] and Aloe vera showed antifungal activity against Aspergillus species [160].
References
- Jarvis, B. (1975). Mycotoxins in food—their occurrence and significance. International Journal of Environmental Studies, 8(1-4), 187- 194.
- Pitt, J. I., Basilico, J. C., Abarca, M. L., & Lopez, C. (2000). Mycotoxins and toxigenic fungi. Sabouraudia, 38(Supplement_1), 41-46.
- Richard, J. L. (2007). Some major mycotoxins and their mycotoxicoses—An overview. International journal of food microbiology, 119(1-2), 3-10.
- Adeyeye, S. A. (2016). Fungal mycotoxins in foods: A review. Cogent Food & Agriculture, 2(1), 1213127.
- Ghannoum, M. A., & Rice, L. B. (1999). Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clinical microbiology reviews, 12(4), 501-517.
- Sequeira, S., Cabrita, E. J., & Macedo, M. F. (2012). Antifungals on paper conservation: An overview. International biodeterioration & biodegradation, 74, 67-86.
- Beyth, N., Houri-Haddad, Y., Domb, A., Khan, W., & Hazan, R. (2015). Alternative antimicrobial approach: nano-antimicrobial materials. Evidence-based complementary and alternative medicine, 2015.
- Brandelli, A., Ritter, A. C., & Veras, F. F. (2017). Antimicrobial Activities of Metal Nanoparticles. In Metal Nanoparticles in Pharma (pp. 337-363). Springer, Cham.
- Zhang, X. (2015). Gold nanoparticles: recent advances in the biomedical applications. Cell biochemistry and biophysics, 72(3), 771-775.
- Rai, M., Ingle, A. P., Birla, S., Yadav, A., & Santos, C. A. D. (2016). Strategic role of selected noble metal nanoparticles in medicine. Critical reviews in microbiology, 42(5), 696-719.
PMid:26089024 - Ogar, A., Tylko, G., & Turnau, K. (2015). Antifungal properties of silver nanoparticles against indoor mould growth. Science of the Total Environment, 521, 305-314.
- Rajeshkumar, S., Malarkodi, C., Vanaja, M., & Annadurai, G. (2016). Anticancer and enhanced antimicrobial activity of biosynthesizd silver nanoparticles against clinical pathogens. Journal of Molecular Structure, 1116, 165-173.
- Davidson, P. M. (2001). Chemical Preservatives and Naturally Antimicrobial Compounds cit Celikel, N. and Kavas, G., 2008, Antimicrobial Properties of Some Essential Oils Against Some Pathogenic Microorganisms. Czech J. Food Sci, 26(3), 174-18.
- Ahmed, S., Ahmad, M., Swami, B. L., & Ikram, S. (2016). A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. Journal of advanced research, 7(1), 17-28.
- Ferrante, M., Sciacca, S., & Conti, G. O. (2012). Carcinogen role of food by mycotoxins and knowledge gap. In Carcinogen. InTech.
- Fung, F., & Clark, R. F. (2004). Health effects of mycotoxins: a toxicological overview. Journal of Toxicology: Clinical Toxicology, 42(2), 217-234.
- Binder, E. M., Tan, L. M., Chin, L. J., Handl, J., & Richard, J. (2007). Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Animal feed science and technology, 137(3-4), 265-282.
- Smith, D. and Onions, A.H.S. (1994). The Preservation and Maintenance of Living Fungi. 2nd edn. IMI Technical Handbooks No. 2. Wallingford, UK: CABI, pp. 122.
- Desjardins, A. E., Hohn, T. M., & McCORMICK, S. P. (1993). Trichothecene biosynthesis in Fusarium species: chemistry, genetics, and significance. Microbiological reviews, 57(3), 595-604.
- Zain, M. E. (2011). Impact of mycotoxins on humans and animals. Journal of Saudi Chemical Society, 15(2), 129-144.
- D’Mello, J.P.F. and MacDonald, A.M.C., 1997. Mycotoxins. Animal Feed Science and Technology 69(1-3),155-166.
- Hossain, M. A., Ahmed, M. S., & Ghannoum, M. A. (2004). Attributes of Stachybotrys chartarum and its association with human disease. Journal of Allergy and Clinical Immunology, 113(2), 200-208.
- Yoko, I. T. O., Peterson, S. W., Donald, T., & Tetsuhisa, G. O. T. O. (2001). Aspergillus pseudotamarii, a new aflatoxin producing species in Aspergillus section Flavi. Mycological Research, 105(02), 233-239.
- Cleveland, T. E., Dowd, P. F., Desjardins, A. E., Bhatnagar, D., & Cotty, P. J. (2003). United States Department Department of Agriculture-Agricultural Research Service research on pre-harvest prevention of mycotoxigenic fungi in US crops (No. CIMMYT.).
- Cotty, P. J. (2006). Biocompetitive exclusion of toxigenic fungi. The mycotoxin factbook: food and feed topics, 179-197.
- Bhat, R., Rai, R. V., & Karim, A. A. (2010). Mycotoxins in food and feed: present status and future concerns. Comprehensive Reviews in Food Science and Food Safety, 9(1), 57-81.
- Williams, J. H., Phillips, T. D., Jolly, P. E., Stiles, J. K., Jolly, C. M., & Aggarwal, D. (2004). Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. The American journal of clinical nutrition, 80(5), 1106-1122.
- Mantle, P. G., & McHugh, K. M. (1993). Nephrotoxic fungi in foods from nephropathy households in Bulgaria. Mycological Research, 97(2), 205- 212.
- Kuiper-Goodman, T., & Scott, P. M. (1989). Risk assessment of the mycotoxin ochratoxin A. Biomedical and environmental sciences: BES, 2(3), 179- 248.
- Kuiper-Goodman, T. (1996). Risk assessment of ochratoxin A: an update. Food additives and Contaminants, 13, 53.
- Pitt, J. I., Basilico, J. C., Abarca, M. L., & Lopez, C. (2000). Mycotoxins and toxigenic fungi. Sabouraudia, 38(Supplement_1), 41-46.
- Refai, M. K., & Hassan, A. A. (2013). Monograph On Mycotoxigenic Fungi and Mycotoxins in food and feeds with synopsis of the authours done on Mycotoxigenic Fungi and Mycotoxins in Foods and Feeds. Cairo, academic edu/Mohamed Refai.
- Samson, R. A., Peterson, S. W., Frisvad, J. C., & Varga, J. (2011). New species in Aspergillus section Terrei. Studies in mycology, 69, 39-55.
- Anjorin, T. S., Salako, E. A., & Makun, H. A. (2013). Control of toxigenic fungi and mycotoxins with phytochemicals: potentials and challenges. In Mycotoxin and Food Safety in Developing Countries. InTech.
- Yassin, M. A., El-Samawaty, A. R. M. A., Moslem, M., Bahkali, A., & Abd-Elsalam, K. (2011). Fungal biota and occurrence of aflatoxigenic Aspergillus in postharvest corn grains. Fresenius Environmental Bulletin, 20(4), 903-909.
- Yassin, M. A., Moslem, M. A., & El-Samawaty, A. E. R. M. (2012). Mycotoxins and non-fungicidal control of corn grain rotting fungi. Journal of plant sciences, 7(3), 96.
- Carlile, M. J., Watkinson, S. C., & Gooday, G. W. (1996). Fungi and Biotechnology-8.
- Moss, M. O., & Frank, J. M. (1985). Influence of the fungicide tridemorph on T-2 toxin production by Fusarium sporotrichioides. Transactions of the British Mycological Society, 84(4), 585-590.
- Pinto, E., Vale-Silva, L., Cavaleiro, C., & Salgueiro, L. (2009). Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. Journal of medical microbiology, 58(11), 1454-1462.
- Aiko, V., & Mehta, A. (2013a). Control of Aspergillus flavus and aflatoxin production with spices in culture medium and rice. World Mycotoxin Journal, 6(1), 43-50.
- Aiko, V., & Mehta, A. (2013b). Inhibitory effect of clove (Syzygium aromaticum) on the growth of Penicillium citrinum and citrinin production. Journal of Food Safety, 33(4), 440-444.
- Bullerman, L. B., Lieu, F. Y., & Seier, S. A. (1977). Inhibition of growth and aflatoxin production by cinnamon and clove oils. Cinnamic aldehyde and eugenol. Journal of Food Science, 42(4), 1107-1109.
- Jayashree, T., & Subramanyam, C. (1999). Anti-aflatoxigenic activity of eugenol is due to inhibition of lipid peroxidation. Letters in Applied Microbiology, 28(3), 179-183.
- Gandomi, H., Misaghi, A., Basti, A. A., Bokaei, S., Khosravi, A., Abbasifar, A., & Javan, A. J. (2009). Effect of Zataria multiflora Boiss. essential oil on growth and aflatoxin formation by Aspergillus flavus in culture media and cheese. Food and chemical toxicology, 47(10), 2397-2400.
- Kumar, A., Shukla, R., Singh, P., & Dubey, N. K. (2010). Chemical composition, antifungal and antiaflatoxigenic activities of Ocimum sanctum essential oil and its safety assessment as plant based antimicrobial. Food and chemical toxicology, 48(2), 539-543.
- Tian, J., Huang, B., Luo, X., Zeng, H., Ban, X., He, J., & Wang, Y. (2012). The control of Aspergillus flavus with Cinnamomum jensenianum -Mazz essential oil and its potential use as a food preservative. Food Chemistry, 130(3), 520-527.
- Bhatnagar, D., Zeringue, H. J. and Cormick, S. P. (1990) Neem leaf extracts inhibit aflatoxin biosynthesis in Aspergillus flavus and parasiticus. In Proceedings of the USDA neem workshop (pp. 118–127). Beltsville, Maryland: US Department of Agriculture.
- Sánchez, E., Heredia, N., & García, S. (2005). Inhibition of growth and mycotoxin production of Aspergillus flavus and Aspergillus parasiticus by extracts of Agave species. International Journal of Food Microbiology, 98(3), 271-279.
- Montes-Belmont, R., & Carvajal, M. (1998). Control of Aspergillus flavus in maize with plant essential oils and their components. Journal of Food Protection, 61(5), 616-619.
- Souza, E. L. D., Lima, E. D. O., Freire, K. R. D. L., & Sousa, C. P. D. (2005). Inhibitory action of some essential oils and phytochemicals on the growth of various moulds isolated from foods. Brazilian archives of Biology and Technology, 48(2), 245-250.
- Magro, A., Carolino, M., Bastos, M., & Mexia, A. (2006). Efficacy of plant extracts against stored products fungi. Revista iberoamericana de micología, 23(3), 176-178.
- Kumar, R., Mishra, A. K., Dubey, N. K., & Tripathi, Y. B. (2007). Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. International journal of food microbiology, 115(2), 159-164.
- Viuda-Martos, M., Ruiz-Navajas, Y., Fernández-López, J., & Pérez-Álvarez, J. (2008). Antifungal activity of lemon (Citrus lemon), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food control, 19(12), 1130-1138.
- Zabka, M., Pavela, R., & Slezakova, L. (2009). Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Industrial Crops and Products, 30(2), 250-253.
- Centeno, S., Calvo, M. A., Adelantado, C., & Figueroa, S. (2010). Antifungal activity of extracts of Rosmarinus officinalis and Thymus vulgaris against Aspergillus flavus and ochraceus. Pakistan journal of biological sciences: PJBS, 13(9), 452-455.
- da Cruz Cabral, L., Pinto, V. F., & Patriarca, A. (2013). Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. International Journal of Food Microbiology, 166(1), 1-14.
- Singh, J., Reen, R. K., & Wiebel, F. J. (1994). Piperine, a major ingredient of black and long peppers, protects against AFB1-induced cytotoxicity and micronuclei formation in H4IIEC3 rat hepatoma cells. Cancer Letters, 86(2), 195-200.
- de Mejía, E. G., Ramos-Gómez, M., & Loarca-Piña, G. (1997). Antimutagenic activity of natural xanthophylls against aflatoxin B1 in Salmonella typhimurium. Environmental and molecular mutagenesis, 30(3), 346-353.
- Rauscher, R., Edenharder, R., & Platt, K. L. (1998). In vitro antimutagenic and in vivo anticlastogenic effects of carotenoids and solvent extracts from fruits and vegetables rich in carotenoids. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 413(2), 129-142.
- Efuntoye, M. O. (1996). Fungi associated with herbal drug plants during storage. Mycopathologia, 136(2), 115-118.
- Efuntoye, M. O. (1999). Mycotoxins of fungal strains from stored herbal plants and mycotoxin contents of Nigerian crude herbal drugs. Mycopathologia, 147(1), 43-48.
- Hell, K., Cardwell, K. F., Setamou, M., & Poehling, H. M. (2000). The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, West Africa. Journal of Stored Products Research, 36(4), 365-382.
- Udoh, J. M., Cardwell, K. F., & Ikotun, T. (2000). Storage structures and aflatoxin content of maize in five agroecological zones of Nigeria. Journal of Stored Products Research, 36(2), 187-201.
- Veerapandian, M., & Yun, K. (2011). Functionalization of biomolecules on nanoparticles: specialized for antibacterial applications. Applied microbiology and biotechnology, 90(5), 1655-1667.
- Vaidyanathan, R., Kalishwaralal, K., Gopalram, S., & Gurunathan, S. N.(2008). The burgeoning therapeutic molecule and its green synthesis. Biotech. Adv.,27: 924, 937.
- Nowack, B., Krug, H. F., & Height, M. (2011). 120 years of nanosilver history: implications for policy makers. Environmental science & technology, 45(4), 1177-1183.
- Jones, S. A., Bowler, P. G., Walker, M., & Parsons, D. (2004). Controlling wound bioburden with a novel silver-containing Hydrofiber® dressing. Wound Repair and Regeneration, 12(3), 288-294.
- Iravani, S., Korbekandi, H., Mirmohammadi, S. V., & Zolfaghari, B. (2014). Synthesis of silver nanoparticles: chemical, physical and biological methods. Research in pharmaceutical sciences, 9(6), 385.
- Mittal, A. K., Bhaumik, J., Kumar, S., & Banerjee, U. C. (2014). Biosynthesis of silver nanoparticles: elucidation of prospective mechanism and therapeutic potential. Journal of colloid and interface science, 415, 39-47.
- Raveendran, P., Fu, J., & Wallen, S. L. (2006). A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chemistry, 8(1), 34-38.
- Gardea-Torresdey, J. L., Gomez, E., Peralta-Videa, J. R., Parsons, J. G., Troiani, H., & Jose-Yacaman, M. (2003). Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir, 19(4), 1357-1361.
- Ahmad, A., Mukherjee, P., Senapati, S., Mandal, D., Khan, M. I., Kumar, R., & Sastry, M. (2003). Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids and surfaces B: Biointerfaces, 28(4), 313-318.
- Kim, S. W., Jung, J. H., Lamsal, K., Kim, Y. S., Min, J. S., & Lee, Y. S. (2012). Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology, 40(1), 53-58.
- Jain, P., & Pradeep, T. (2005). Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnology and bioengineering, 90(1), 59-63.
- Mohanpuria, P., Rana, N. K., & Yadav, S. K. (2008). Biosynthesis of nanoparticles: technological concepts and future applications. Journal of nanoparticle research, 10(3), 507-517.
- Oberdörster, G., Oberdörster, E., & Oberdörster, J. (2005). Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113: 823–839.
- Dipankar, C., & Murugan, S. (2012). The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids and Surfaces B: Biointerfaces, 98, 112-119.
- Rai, M., Yadav, A., & Gade, A. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnology advances, 27(1), 76-83.
- Rai, M., Kon, K., Ingle, A., Duran, N., Galdiero, S., & Galdiero, M. (2014). Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Applied microbiology and biotechnology, 98(5), 1951-1961.
- Morones, J. R., Elechiguerra, J. L., Camacho, A., Holt, K., Kouri, J. B., Ramírez, J. T., & Yacaman, M. J. (2005). The bactericidal effect of silver nanoparticles. Nanotechnology, 16(10), 2346.
- Shahverdi, A. R., Fakhimi, A., Shahverdi, H. R., & Minaian, S. (2007). Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine: Nanotechnology, Biology and Medicine, 3(2), 168-171.
- Thomas, R., Nair, A. P., Soumya, K. R., Mathew, J., & Radhakrishnan, E. K. (2014). Antibacterial activity and synergistic effect of biosynthesized AgNPs with antibiotics against multidrug-resistant biofilm-forming coagulase-negative staphylococci isolated from clinical samples. Applied biochemistry and biotechnology, 173(2), 449-460.
- Reddy, T. R. K., & Kim, H. J. (2016). Facile synthesis of silver nanoparticles and its antibacterial activity against Escherichia coli and unknown bacteria on mobile phone touch surfaces/computer keyboards. Applied Physics A, 122(7), 1-10.
- Lara, H. H., Ayala-Núnez, N. V., Turrent, L. D. C. I., & Padilla, C. R. (2010). Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World Journal of Microbiology and Biotechnology, 26(4), 615- 62.
- Zhang, Y. H., & Qi, H. J. (2008). Composite fluorocarbon/ZnO films prepared by RF magnetron sputtering of Zn and PTFE. Surface and Coatings Technology, 202(12), 2612-2615.
- Kim, K. J., Sung, W. S., Moon, S. K., Choi, J. S., Kim, J. G., & Lee, D. G. (2008). Antifungal effect of silver nanoparticles on dermatophytes. J Microbiol Biotechnol, 18(8), 1482-1484.
- Panáček, A., Kolář, M., Večeřová, R., Prucek, R., Soukupová, J., Kryštof, V., … & Kvítek, L. (2009). Antifungal activity of silver nanoparticles against Candida spp. Biomaterials, 30(31), 6333-6340.
- Azizi, Z., Pourseyedi, S., Khatami, M., & Mohammadi, H. (2016). Stachys lavandulifolia and Lathyrus sp. mediated for green synthesis of silver nanoparticles and evaluation its antifungal activity against dothiorella sarmentorum. Journal of Cluster Science, 27(5), 1613-1628.
- Gajbhiye, M., Kesharwani, J., Ingle, A., Gade, A., & Rai, M. (2009). Fungus mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine: Nanotechnology, Biology and Medicine, 5(4), 382-386.
- Rathnayake, W. G. I. U., Ismail, H., Baharin, A., Darsanasiri, A. G. N. D., & Rajapakse, S. (2012). Synthesis and characterization of nano silver based natural rubber latex foam for imparting antibacterial and anti-fungal properties. Polymer Testing, 31(5), 586-592.
- Mousavi, S. A. A., & Pourtalebi, S. (2015). Inhibitory Effects of Silver Nanoparticles on Growth and Aflatoxin B1 Production by Aspergillus Parasiticus. Iranian journal of medical sciences, 40(6), 501.
- Matei, A., Cornea, C. P., Matei, S., Matei, G. M., Cogălniceanu, G., & Rodino,S. (2015). Biosynthesis of silver nanoparticles using culture filtrates of lactic acid bacteria and analysis of antifungal activity. Digest Journal of Nanomaterials and Biostructures, 10(4), 1201-1207.
- Dahl, J. A., Maddux, B. L., & Hutchison, J. E. (2007). Toward greener nanosynthesis. Chemical reviews, 107(6), 2228-2269.
- Kharissova, O. V., Dias, H. R., Kharisov, B. I., Pérez, B. O., & Pérez, V. M. J. (2013). The greener synthesis of nanoparticles. Trends in biotechnology, 31(4), 240-248.
- Mallikarjuna, K., Narasimha, G., Dillip, G. R., Praveen, B., Shreedhar, B., Lakshmi, C. S., … & Raju, B. D. P. (2011). Green synthesis of silver nanoparticles using Ocimum leaf extract and their characterization. Digest Journal of Nanomaterials and Biostructures, 6(1), 181-186.
- MubarakAli, D., Thajuddin, N., Jeganathan, K., & Gunasekaran, M. (2011). Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids and Surfaces B: Biointerfaces, 85(2), 360-365.
- Saxena, A., Tripathi, R. M., Zafar, F., & Singh, P. (2012). Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Materials letters, 67(1), 91-94.
- Santhoshkumar, T., Rahuman, A. A., Rajakumar, G., Marimuthu, S., Bagavan, A., Jayaseelan, C., … & Kamaraj, C. (2011). Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitology research, 108(3), 693-702.
- Kouvaris, P., Delimitis, A., Zaspalis, V., Papadopoulos, D., Tsipas, S. A., & Michailidis, N. (2012). Green synthesis and characterization of silver nanoparticles produced using Arbutus unedo leaf extract. Materials Letters, 76, 18-20.
- Ponarulselvam, S., Panneerselvam, C., Murugan, K., Aarthi, N., Kalimuthu, K., & Thangamani, S. (2012). Synthesis of silver nanoparticles using leaves of Catharanthus roseus G. Don and their antiplasmodial activities. Asian Pacific Journal of Tropical Biomedicine, 2(7), 574-580.
- Vijayakumar, M., Priya, K., Nancy, F. T., Noorlidah, A., & Ahmed, A. B. A. (2013). Biosynthesis, characterisation and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Industrial Crops and Products, 41, 235-240.
- Logeswari, P., Silambarasan, S., & Abraham, J. (2013). Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties. Scientia Iranica, 20(3), 1049-1054.
- Veerasamy, R., Xin, T. Z., Gunasagaran, S., Xiang, T. F. W., Yang, E. F. C., Jeyakumar, N., & Dhanaraj, S. A. (2011). Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. Journal of Saudi Chemical Society, 15(2), 113-120.
- Abdel-Aziz, M. S., Shaheen, M. S., El-Nekeety, A. A., & Abdel-Wahhab, M. A. (2014). Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. Journal of Saudi Chemical Society, 18(4), 356-363.
- Bindhu, M. R., & Umadevi, M. (2013). Synthesis of monodispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 101, 184-190.
- Das, J., Das, M. P., & Velusamy, P. (2013). Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 104, 265-270.
- Ahmed, S., Ahmad, M., Swami, B. L., & Ikram, S. (2016). Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. Journal of Radiation Research and Applied Sciences, 9(1), 1-7.
- Khalil, M. M., Ismail, E. H., El-Baghdady, K. Z., & Mohamed, D. (2014). Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arabian Journal of Chemistry, 7(6), 1131-1139.
- Jancy, M. E., & Inbathamizh, L. (2012). Green synthesis and characterization of nano silver using leaf extract of Morinda pubescens. Asian J Pharm Clin Res, 5(Suppl 1), 159-162.
- Umashankari, J., Inbakandan, D., Ajithkumar, T. T., & Balasubramanian, T. (2012). Mangrove plant, Rhizophora mucronata (Lamk, 1804) mediated one pot green synthesis of silver nanoparticles and its antibacterial activity against aquatic pathogens. Aquatic biosystems, 8(1), 11.
- Vivek, M., Kumar, P. S., Steffi, S., & Sudha, S. (2011). Biogenic silver nanoparticles by Gelidiella acerosa extract and their antifungal effects. Avicenna Journal of Medical Biotechnology, 3(3), 143.
- Saifuddin, N., Wong, C. W., & Yasumira, A. A. (2009). Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. Journal of Chemistry, 6(1), 61-70.
- Malarkodi, C., Rajeshkumar, S., Paulkumar, K., Vanaja, M., Jobitha, G. D. G., & Annadurai, G. (2013). Bactericidal activity of bio mediated silver nanoparticles synthesized by Serratia nematodiphila. Drug Invention Today, 5(2), 119-125.
- Zaki, S., El Kady, M. F., & Abd-El-Haleem, D. (2011). Biosynthesis and structural characterization of silver nanoparticles from bacterial isolates. Materials research bulletin, 46(10), 1571-1576.
- Kalishwaralal, K., Deepak, V., Pandian, S. R. K., Kottaisamy, M., BarathManiKanth, S., Kartikeyan, B., & Gurunathan, S. (2010). Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids and Surfaces B: Biointerfaces, 77(2), 257-262.
- Dakhil, A. S. (2017). Biosynthesis of silver nanoparticle (AgNPs) using Lactobacillus and their effects on oxidative stress biomarkers in rats. Journal of King Saud University-Science, 29(4), 462-467.
- Shahverdi, A. R., Fakhimi, A., Shahverdi, H. R., & Minaian, S. (2007). Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine: Nanotechnology, Biology and Medicine, 3(2), 168-171.
- Bhat, M. A., Nayak, B. K., & Nanda, A. (2015). Evaluation of bactericidal activity of biologically synthesised Silver Nanoparticles from Candida albicans in combination with Ciprofloxacin. Materials Today: Proceedings, 2(9), 4395-4401.
- Vigneshwaran, N., Ashtaputre, N. M., Varadarajan, P. V., Nachane, R. P., Paralikar, K. M., & Balasubramanya, R. H. (2007). Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Materials letters, 61(6), 1413-1418.
- Bhainsa, K. C., & D’souza, S. F. (2006). Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids and surfaces B: Biointerfaces, 47(2), 160-164.
- Singh, T., Jyoti, K., Patnaik, A., Singh, A., Chauhan, R., & Chandel, S. S. (2017). Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. Journal of Genetic Engineering and Biotechnology, 15(1), 31-39.
- Majeed, S., bin Abdullah, M. S., Dash, G. K., Ansari, M. T., & Nanda, A. (2016). Biochemical synthesis of silver nanoprticles using filamentous fungi Penicillium decumbens (MTCC-2494) and its efficacy against A-549 lung cancer cell line. Chinese journal of natural medicines, 14(8), 615-620.
- Shaligram, N. S., Bule, M., Bhambure, R., Singhal, R. S., Singh, S. K., Szakacs, G., & Pandey, A. (2009). Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process biochemistry, 44(8), 939-943.
- Nayak, B. K., Nanda, A., & Prabhakar, V. (2018). Biogenic synthesis of silver nanoparticle from wasp nest soil fungus, Penicillium italicum and its analysis against multi drug resistance pathogens. Biocatalysis and agricultural biotechnology, 16, 412-418.
- Elgorban, A. M., El-Samawaty, A. E. R. M., Abd-Elkader, O. H., Yassin, M. A., Sayed, S. R., Khan, M., & Adil, S. F. (2017). Bioengineered silver nanoparticles using Curvularia pallescens and its fungicidal activity against Cladosporium fulvum. Saudi Journal of Biological Sciences, 24(7), 1522-1528.
- Balakumaran, M. D., Ramachandran, R., & Kalaichelvan, P. T. (2015). Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiological research, 178, 9-17.
- Sarsar, V., Selwal, M. K., & Selwal, K. K. (2015). Biofabrication, characterization and antibacterial efficacy of extracellular silver nanoparticles using novel fungal strain of Penicillium atramentosumJournal of Saudi Chemical Society, 19(6), 682-688.
- Yassin, M. A., El-Samawaty, A. E. R. M., Dawoud, T. M., Abd-Elkader, O. H., Al Maary, K. S., Hatamleh, A. A., & Elgorban, A. M. (2017). Characterization and anti-Aspergillus flavus impact of nanoparticles synthesized by Penicillium citrinum. Saudi journal of biological sciences, 24(6), 1243-1248.
- Bocate, K. P., Reis, G. F., de Souza, P. C., Junior, A. G. O., Durán, N., Nakazato, G., … & Panagio, L. A. (2018). Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. International Journal of Food Microbiology.
- Bocate, K. P., Reis, G. F., de Souza, P. C., Junior, A. G. O., Durán, N., Nakazato, G., … & Panagio, L. A. (2018). Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. International Journal of Food Microbiology.
- Husseiny, S. M., Salah, T. A., & Anter, H. A. (2015). Biosynthesis of size controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. Beni-Suef University Journal of Basic and Applied Sciences, 4(3), 225-231.
- Sinha, S. N., Paul, D., Halder, N., Sengupta, D., & Patra, S. K. (2015). Green synthesis of silver nanoparticles using fresh water green alga Pithophora oedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Applied Nanoscience, 5(6), 703-709.
- Kathiraven, T., Sundaramanickam, A., Shanmugam, N., & Balasubramanian, T. (2015). Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Applied Nanoscience, 5(4), 499-504.
- Barwal, I., Ranjan, P., Kateriya, S., & Yadav, S. C. (2011). Cellular oxido-reductive proteins of Chlamydomonas reinhardtii control the biosynthesis of silver nanoparticles. Journal of nanobiotechnology, 9(1), 56.
- Kannan, R. R. R., Arumugam, R., Ramya, D., Manivannan, K., & Anantharaman, P. (2013). Green synthesis of silver nanoparticles using marine macroalga Chaetomorpha linum. Applied Nanoscience, 3(3), 229-233.
- Prasad, T. N., Kambala, V. S. R., & Naidu, R. (2013). Phyconanotechnology: synthesis of silver nanoparticles using brown marine algae Cystophora moniliformis and their characterisation. Journal of applied phycology, 25(1), 177-182.
- Dhanalakshmi, P. K., Azeez, R., Rekha, R., Poonkodi, S., & Nallamuthu, T. (2012). Synthesis of silver nanoparticles using green and brown seaweeds. Phykos, 42(2), 39-45.
- Kumar, P., Selvi, S. S., & Govindaraju, M. (2013). Seaweed-mediated biosynthesis of silver nanoparticles using Gracilaria corticata for its antifungal activity against CandidaApplied Nanoscience, 3(6), 495-500.
- Salari, Z., Danafar, F., Dabaghi, S., & Ataei, S. A. (2016). Sustainable synthesis of silver nanoparticles using macroalgae Spirogyra varians and analysis of their antibacterial activity. Journal of Saudi Chemical Society, 20(4), 459-464.
- Jegadeeswaran, P., Shivaraj, R., & Venckatesh, R. (2012). Green synthesis of silver nanoparticles from extract of Padina tetrastromaticaDigest Journal of Nanomaterials and Biostructures, 7(3), 991-998.
- Venkatpurwar, V., & Pokharkar, V. (2011). Green synthesis of silver nanoparticles using marine polysaccharide: Study of in-vitro antibacterial activity. Materials Letters, 65(6), 999-1002.
- Shankar, S. S., Rai, A., Ahmad, A., & Sastry, M. (2004). Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. Journal of colloid and interface science, 275(2), 496-502.
- Ravindra, S., Mohan, Y. M., Reddy, N. N., & Raju, K. M. (2010). Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via “Green Approach”. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 367(1), 31-40
- Medda, S., Hajra, A., Dey, U., Bose, P., & Mondal, N. K. (2015). Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Applied Nanoscience, 5(7), 875- 880.
- Hernandez, L. G., Islas, D. A., Guerrero, M. F., Ortega, P. R., & Lechuga, L. G. (2015). Synthesis of Bimetallic Nanoparticles Urchin-Like with Ricinus Communis Leaf Extract. In TMS 2015 144th Annual Meeting & Exhibition (pp. 1113-1118). Springer, Cham.
- Augustine, R., Kalarikkal, N., & Thomas, S. (2014). A facile and rapid method for the black pepper leaf mediated green synthesis of silver nanoparticles and the antimicrobial study. Applied Nanoscience, 4(7), 809-818.
- Vijaya, P. P., Rekha, B., Mathew, A. T., Ali, M. S., Yogananth, N., Anuradha, V., & Parveen, P. K. (2014). Antigenotoxic effect of green-synthesised silver nanoparticles from Ocimum sanctum leaf extract against cyclophosphamide induced genotoxicity in human lymphocytes—in vitro. Applied Nanoscience, 4(4), 415-420.
- Velmurugan, P., Anbalagan, K., Manosathyadevan, M., Lee, K. J., Cho, M., Lee, S. M., … & Oh, B. T. (2014). Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens. Bioprocess and biosystems engineering, 37(10), 1935-1943.
- Moyo, M., Gomba, M., & Nharingo, T. (2015). Afzelia quanzensis bark extract for green synthesis of silver nanoparticles and study of their antibacterial activity. International Journal of Industrial Chemistry, 6(4), 329-338.
- Mubarak Ali, D., Thajuddin, N., Jeganathan, K., & Gunasekaran, M. (2011). Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids and Surfaces B: Biointerfaces, 85(2), 360-365.
- Rashid, M. M. O., Ferdous, J., Banik, S., Islam, M. R., Uddin, A. M., & Robel, F. N. (2016). Anthelmintic activity of silver-extract nanoparticles synthesized from the combination of silver nanoparticles and charantia fruit extract. BMC Complementary and Alternative Medicine, 16(1), 242.
- Dauthal, P., & Mukhopadhyay, M. (2013). In-vitro free radical scavenging activity of biosynthesized gold and silver nanoparticles using Prunus armeniaca (apricot) fruit extract. Journal of nanoparticle research, 15(1), 1-11.
- Khatami, M., Pourseyedi, S., Khatami, M., Hamidi, H., Zaeifi, M., & Soltani, L. (2015). Synthesis of silver nanoparticles using seed exudates of Sinapis arvensis as a novel bioresource, and evaluation of their antifungal activity. Bioresources and Bioprocessing, 2(1), 1.
- Zia, F., Ghafoor, N., Iqbal, M., & Mehboob, S. (2016). Green synthesis and characterization of silver nanoparticles using Cydonia oblong seed extract. Applied Nanoscience, 1-7.
- Basu, S., Maji, P., & Ganguly, J. (2016). Rapid green synthesis of silver nanoparticles by aqueous extract of seeds of Nyctanthes arbor- tristis. Applied Nanoscience, 6(1), 1-5.
- Ahmed, S., Ahmad, M., Swami, B. L., & Ikram, S. (2016). A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. Journal of advanced research, 7(1), 17-28.
- Bankar, A., Joshi, B., Kumar, A. R., & Zinjarde, S. (2010). Banana peel extract mediated novel route for the synthesis of palladium nanoparticles. Materials Letters, 64(18), 1951-1953.
- Govindaraju, K., Tamilselvan, S., Kiruthiga, V., & Singaravelu, G. (2010). Biogenic silver nanoparticles by Solanum torvum and their promising antimicrobial activity. Journal of Biopesticides, 3(1), 394-399.
- Singh, A., Jain, D., Upadhyay, M. K., Khandelwal, N., & Verma, H. N. (2010). Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activities. Dig J Nanomater Bios, 5(2), 483-489.
- Medda, S., Hajra, A., Dey, U., Bose, P., & Mondal, N. K. (2015). Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Applied Nanoscience, 5(7), 875-880.